Published by BANG Showbiz English Freddie Mercury stopped taking the drugs that were keeping him alive two weeks before he … [Read more...] about ‘Freddie Mercury The Final Act’ Covers Singers Final Years, Done His Way. Bandmates, Assistants Detail The Joy, Strength , Stigma For New Doc
AIDS/HIV
Billy Porter Pose: Found Growth Playing Pray There’s ‘a whole generation of us who haven’t really been able to process what happened.’
Published by BANG Showbiz English Billy Porter's role in 'Pose' helped him "heal" from his "trauma and grief". The … [Read more...] about Billy Porter Pose: Found Growth Playing Pray There’s ‘a whole generation of us who haven’t really been able to process what happened.’
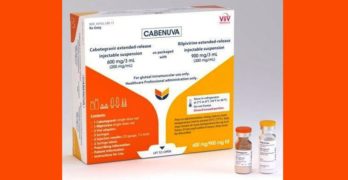
FDA Approves First Once-a-Month Injectable Drug Regimen for People Living with HIV
The FDA this week approved the use of once-a-month injectable Cabenuva for people living with HIV. The FDA reports: "The U.S. Food and Drug … [Read more...] about FDA Approves First Once-a-Month Injectable Drug Regimen for People Living with HIV
Olly Alexander Refuses to Believe Reports of a Virus Targeting Gay Men in ‘It’s a Sin’ Clip: WATCH
Olly Alexander shared a new clip from It's a Sin, the upcoming gay-themed drama from Queer As Folk creator Russell T. Davies about Britain’s AIDS … [Read more...] about Olly Alexander Refuses to Believe Reports of a Virus Targeting Gay Men in ‘It’s a Sin’ Clip: WATCH
Groundbreaking ‘Red Hot + Blue’ Album Returns on World AIDS Day to Mark Org’s 30th Anniversary: WATCH
PBS Newshour took a look back at the Red Hot organization's 30-year-fight against AIDS. Readers of a certain age will know the organization best … [Read more...] about Groundbreaking ‘Red Hot + Blue’ Album Returns on World AIDS Day to Mark Org’s 30th Anniversary: WATCH
Timothy Ray Brown (aka ‘The Berlin Patient’), the First Person Cured of HIV, Dies of Cancer at 54
Timothy Ray Brown, the first man cured of HIV through stem cell transplants who was known for many years as "the Berlin patient," has died of cancer … [Read more...] about Timothy Ray Brown (aka ‘The Berlin Patient’), the First Person Cured of HIV, Dies of Cancer at 54
Seth Meyers and Cynthia Nixon Compare ‘Black Lives Matter’ Protests to the Early Days of the AIDS Crisis: WATCH
Cynthia Nixon sat down with Seth Meyers on Late Night Wednesday to talk about the changing political climate since her run for New York governor and … [Read more...] about Seth Meyers and Cynthia Nixon Compare ‘Black Lives Matter’ Protests to the Early Days of the AIDS Crisis: WATCH
Trump Praises Scientists for ‘AIDS Vaccine’ That Doesn’t Exist Amid Another Lying, Rambling Self-Indulgent Speech: WATCH
Donald Trump gave a speech in the Rose Garden on Tuesday during which he praised scientists for an AIDS vaccine that does not exist. Said Trump: … [Read more...] about Trump Praises Scientists for ‘AIDS Vaccine’ That Doesn’t Exist Amid Another Lying, Rambling Self-Indulgent Speech: WATCH
Activist, Author and Playwright Larry Kramer Has Died at 84
Activist, author, and playwright Larry Kramer has died at the age of 84. Kramer was a lifelong AIDS activist, raising his voice when others wouldn't. … [Read more...] about Activist, Author and Playwright Larry Kramer Has Died at 84
Injectable Long-Acting PrEP Shown to Be 69 Percent More Effective at Averting HIV Infection Than Truvada
A new injectable form of PrEP (pre-exposure prophylaxis) from ViiV Healthcare administered every two months has been shown to be 69 percent more … [Read more...] about Injectable Long-Acting PrEP Shown to Be 69 Percent More Effective at Averting HIV Infection Than Truvada